potassium ion electrons|Potassium : Bacolod In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron config.
Hello everybody, here we are today with Wordalot, new exciting quiz for Android. This is a brand new game developed by MAG Interactive who have also developed Wordbrain, Wordbrain Themes and Ruzzle. This game is not like the Wordbrain game which all you need to do was to guess what words where shown in each level and slide the finger .
PH0 · WebElements Periodic Table » Potassium » the essentials
PH1 · Potassium – Protons – Neutrons – Electrons – Electron
PH2 · Potassium Electron Configuration (K) with Orbital
PH3 · Potassium
PH4 · K+ Electron Configuration (Potassium Ion)
PH5 · How to find Protons & Electrons for the K+ (Potassium ion)
PH6 · How to Write the Electron Configuration for Potassium (K)
PH7 · Electron Configuration for Potassium (K, K+ ion)
PH8 · Chemistry of Potassium (Z=19)
The Warriors' Guild is a large building located in the west of Burthorpe, founded by Harrallak Menarous.Only players with a combined Attack and Strength level of at least 130 (or level 99 in either skill), are allowed entrance by the guild's guard Ghommal.Temporary boosts cannot be used.. Inside the guild, players may play various minigames related to .
potassium ion electrons*******Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. This electron configuration shows that the last shell of the potassium atom has an unpaired electron. So the valency of . Tingnan ang higit paThe total number of electrons in potassiumis nineteen. These electrons are arranged according to specific rules in different . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paAfter arranging the electrons, it is seen that the last shell of the potassium atom has an electron. Therefore, the valence electrons of potassiumare one. The elements . Tingnan ang higit pa In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron config. 791K subscribers. Subscribed. 155. 20K views 3 years ago. In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the . There are 25 known isotopes of potassium, three of which occur naturally: 39K (93.3%), 40K (0.0117%), and 41K (6.7%). Potassium-39 is composed of 19 protons, 20 .In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll put .
In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge (which combines with anions to form .
The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to attract electrons towards itself, . Important compounds of potassium include potassium hydroxide (used in some drain cleaners), potassium superoxide, \(KO_2\), which is used in respiratory equipment and .
Potassium atoms have 19 electrons and the shell structure is 2.8.8.1. The ground state electronic configuration of neutral potassium is [ Ar ]. 4s1 and the term symbol of potassium is 2S1/2. . Electron Configuration for Potassium Ion. You can see the electron configuration for K Ion in the picture below; How Many Valence Electrons Are in K? The potassium Number of Valence Electron is nineteen since .
potassium ion electrons Potassium Electronic Configuration of Anions. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Thus, it is simple to determine the charge on such a negative ion: The charge is equal to the number of electrons that must be gained to fill the s and p . If, however, there are fewer electrons than protons as in the case of the potassium ion, the net charge is positive since there is one more positive proton than negative electrons [(+19) + (−18) = +1]. Positive ions are cations. When there are more electrons than protons, as in the case of the sulfur ion, the net charge is negative [(+16 .
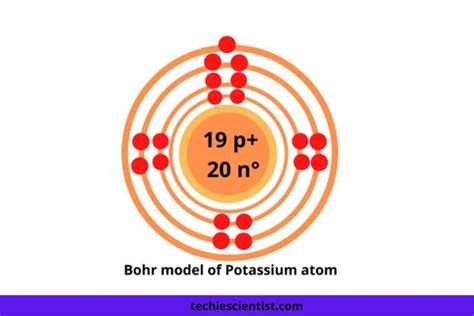
Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . For example, iron (1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2) forms the ion Fe 2+ (1s 2 2s 2 2p 6 3s 2 . In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron config.
potassium ion electrons In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron config. The electron configuration of potassium ion(K +) is 1s 2 2s 2 2p 6 3s 2 3p 6. The electron configuration of potassium-ion shows that the potassium ion has three shells and the last shell has eight electrons. This electron configuration shows that the potassium atom has acquired the electron configuration of argon.Potassium All you are allowed to add to this equation are water, hydrogen ions and electrons. Adding water is obviously unhelpful: if water is added to the right-hand side to supply extra hydrogen atoms, an additional oxygen atom is needed on the left. . Potassium dichromate(VI) solution acidified with dilute sulfuric acid is used to oxidize ethanol .
With arrows, illustrate the transfer of electrons to form potassium sulfide from K atoms and S atoms. . The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom (Z = 8) has eight electrons, and if it gains two electrons it will become an .
In the case of the 19th element, the color is pale lavender. Like sodium ions, the presence of potassium ions in the body is essential for the correct function of many cells. Table \(\PageIndex{1}\): Basic Chemical and Physical Properties; . Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: Notable Reactions with Phosphorus.
First Ionization Energy of Potassium. First Ionization Energy of Potassium is 4.3407 eV. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.. X + energy → X + + e −. where X is any atom or molecule capable of being ionized, X + is that atom or molecule with an electron removed (positive ion), and e − is .For anions, the charge tells how many extra electrons there are compared to the number of protons. Recall, this pattern for the formation of anions and cations: Metals tend to lose electron(s) and become cations (positively charged ions). .
For ions of the same charge (e.g. in the same group) the size increases as we go down a group in the periodic table . Consider the following collection of ions: ion electrons protons; O 2-10: 8: F-10: 9: Na + 10: 11: Mg 2 . The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another. In electron transfer, the number of electrons lost must equal the number of electrons gained. We saw this in the formation of NaCl.

For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Cations. As stated previously, cations are positively-charged ions that are most often formed when metals, which are found on the left side of the periodic table, lose valence electrons. For example, consider calcium (Ca). An atom of calcium contains 20 protons, because its atomic number is 20, and 20 electrons, in order to be net-neutral. On the right, the chloride ion has 18 electrons and has a 1− charge. Neutral chlorine atom on left has 17 protons and 17 electrons. Sodium ion on right has 17 protons and 18 electrons, with a -1 overall charge. The names for positive and negative ions are pronounced CAT-eye-ons and ANN-eye-ons, respectively. All of the alkali metals have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge – a cation, which combines with anions to form salts. Naturally occurring potassium is .
ocn モバイル oneご利用中の方に、ocnオンラインショップで使える割引クーポンを配布中!5,000円引きでお得に機種変更ができるチャンス!「ocnでんわ」や「あんしんモバイルパック」など各種オプションのご利用お申し込みも可能です。
potassium ion electrons|Potassium